Abstract
Background: Cytokine release syndrome (CRS) is a potentially life-threatening toxicity caused by immune activation. CRS can be triggered non-specifically by T-cell engaging therapies. Komanduri et al. (ASH 2021) outlined a model based on eight baseline factors (CRS-risk score; CRS-RS) which allowed accurate classification of risk for Grade ≥2 CRS upon treatment with glofitamab.
Glofitamab is a CD20xCD3 T-cell-engaging bispecific monoclonal antibody with a novel 2:1 (CD20:CD3) configuration that has shown promising efficacy with a manageable safety profile in NP30179 (NCT03075696), an ongoing Phase I/II dose-finding study of glofitamab in pts with relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL; Dickinson et al. ASCO 2022). While CRS is observed with glofitamab, cases typically occur with Grade 1/2 severity (per ASTCT grading; Lee et al. Biol Blood Marrow Transplant 2019) mostly in the first cycle (C).
Here, we introduce a streamlined model based on five baseline factors (CRS-RS.5p) derived from the original CRS-RS, an ongoing non-interventional study (GO43921) to assess the performance of CRS-RS.5p, and the potential application of the risk score in a clinical setting.
Methods: In both models, using a weighted sum of baseline clinical, laboratory and imaging parameters, pts are assigned a risk score as a continuous measure for developing CRS after the first dose of glofitamab. Using a defined cut-off, pts are classified as low or high risk of CRS. The original CRS-RS model parameters were: age >64 years at study entry, lactate dehydrogenase pre-anti-CD20 treatment >280 U/L, white blood cell count pre-anti-CD20 treatment >4.5x109 cells/L, Ann Arbor Stage III/IV at study entry, sum of the product of the perpendicular diameters at study entry ≥3000mm2, cardiac comorbidity, bone marrow infiltration, and atypical or leukemic cells in peripheral blood at study entry. The simplified CRS-RS.5p concentrates on the first five parameters utilizing the same weighting scheme as in CRS-RS.
The primary objective of the GO43921 study is to determine if a five-baseline-parameter model can predict Grade ≥2 CRS events after the first dose of glofitamab. Seven studies were identified in which pts with aggressive NHL (first line and R/R, excluding mantle cell lymphoma) were treated with glofitamab step-up dosing as either monotherapy or part of combination therapy. In most studies, pts are pretreated with 1000mg obinutuzumab, with intravenous glofitamab being administered on Days (D) 1 (2.5mg) and 8 (10mg) of C1, then at the target dose (30mg) from C2D1 onwards. Several combination studies started glofitamab step-up dosing after multiple cycles of R-CHOP (glofitamab D8 [2.5mg], D15 [10mg], target dose D8 [30mg] in the following cycle). Prospective data has been collected since December 2021, and retrospective data from included studies were analyzed to increase the sample of Grade ≥2 CRS events.
Here, CRS-RS.5p is compared with CRS-RS for the NP30179 dataset, and CRS-RS.5p performance in the ongoing GO43921 study is analyzed.
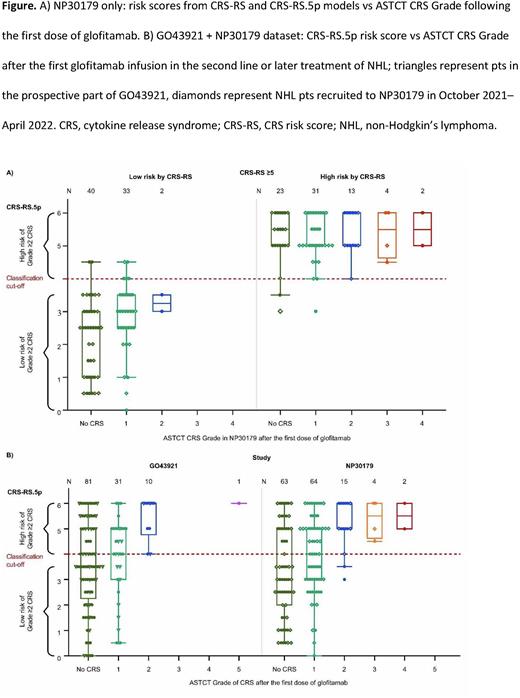
Results: In NP30179, data acquired since the introduction of CRS-RS (n=44) showed that CRS-RS and CRS-RS.5p identified as high risk the same pts who experienced Grade ≥2 events (Figure A; four Grade 2, one Grade 3). In the whole NP30179 validation cohort, both CRS-RS and CRS-RS.5p achieved negative predictive values of 0.97 (n=148, Standard error=0.02). For GO43921, at the clinical cut-off date (June 6, 2022), the CRS-RS.5p model had predicted all 11 of the Grade ≥2 CRS events in the second-line or later setting for pts with aggressive NHL (n=123, with data collected retrospectively for 82 pts; Figure B). The association of the CRS-RS.5p score with the ASTCT CRS Grade after the first dose of glofitamab was similar across all available data sets from NP30179 and GO43921 (logistic regression; p<0.001 for NP30179, p=0.004 for GO43921). No influence of steroid premedication class on CRS-RS performance was observed. A low-risk group (CRS-RS.5p <4.0) comprised 48% of pts in NP30179 and 46% in GO43921.
Conclusions: Data from the CRS-RS.5p model showed identical performance with the previous 8-parameter CRS-RS model in predicting which pts with aggressive NHL are most at risk of Grade ≥2 CRS after the first dose of glofitamab. With reduced complexity, the CRS-RS.5p has a potential application as a practical, clinical decision tool to guide hospitalization for pts at high risk of Grade ≥2 CRS.
Disclosures
Belousov:F. Hoffmann La Roche, Ltd.: Current Employment, Current holder of stock options in a privately-held company. Dixon:Roche Products Limited: Current Employment; Roche: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Relf:Roche Products Limited: Current Employment; Roche, F-Star Therapeutics: Current equity holder in publicly-traded company; Roche, F-Star Therapeutics and Harpoon Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months. Tandon:Roche: Current Employment, Current equity holder in publicly-traded company.
OffLabel Disclosure:
Glofitamab is a full-length, humanized immunoglobulin G1 bispecific monoclonal antibody with a 2:1 (CD20:CD3) configuration that facilitates bivalent binding to CD20 on B cells, and monovalent binding to CD3 on T cells. Glofitamab redirects T cells to eliminate normal and malignant B cells. Glofitamab is an investigational agent. Obinutuzumab (Gazyva) is a CD20-directed cytolytic antibody indicated: in combination with chlorambucil, for the treatment of pts with previously untreated CLL; in combination with bendamustine followed by obinutuzumab monotherapy, for the treatment of pts with FL who relapsed after, or are refractory to, a rituximab-containing regimen; in combination with chemo followed by obinutuzumab monotherapy in pts achieving at least a PR, for the treatment of adult pts with previously untreated stage II bulky, III or IV FL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal